The research in my lab aims to better understand the biology underlying the genesis, progression, and therapy resistance of upper gastrointestinal (GI) tract cancers, in particular pancreatic and esophageal cancer. Theses malignancies are highly lethal and it is clear that we know too little about these diseases to effectively treat them. Models in my lab to study these processes range from cell culture systems of varying complexity, to mouse experiments, biobanking and the analysis of predictive and prognostic markers and profiles in large cohorts.
cancer stroma
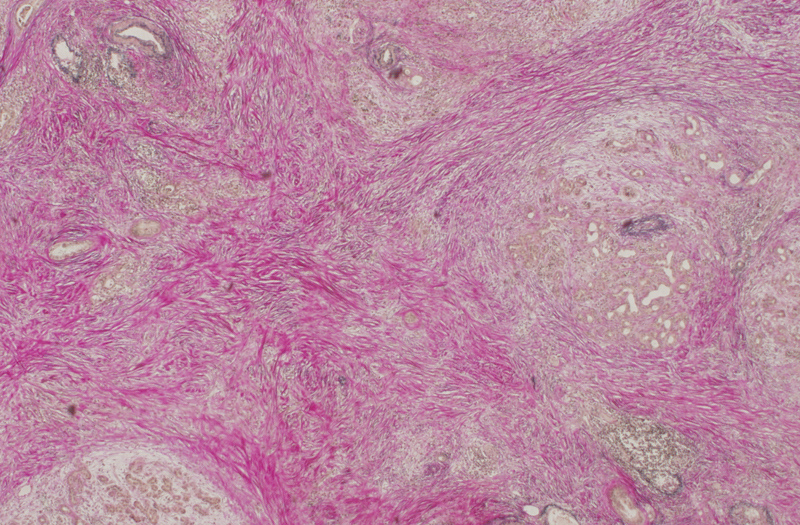
One of the most typical features of upper GI tract tumors is the abundance of non-cancerous cells and material that surrounds the cancer cells. The collective of these entities is called the stroma. A large body of preclinical work has demonstrated that this stroma can, for example, limit drug delivery and promote invasiveness of the tumor cells. Other studies however, have shown a tumor-limiting role for the stroma instead. Our research aims to identify which signals that mediate critical tumor-stroma crosstalk, and whether we can use this to target tumor growth and drug resistance.
In the microscope image of a pancreatic tumor shown below, non-cancerous cells and tissue are stained pink by using a so-called Van Gieson‘s stain. What is immediately apparent, is the excess of this material relative to the actual cancer cells that grow in the brownish clusters. Despite (or possibly because of) the presence of relatively few cancer cells, these tumors are extremely lethal.
This remarkable tumor biology has sparked the development of therapeutics that target the stroma, and these have recently come to the clinic. Despite encouraging results for some of these therapeutics, a need to better understand which patients benefit most from stroma-targeting treatments has become apparent. Using relatively specific drugs in unselected populations can mask a potentially encouraging outcome. Our lab is developing blood-borne markers that can stratify patients for these treatments and hopefully increase their efficacy or spare patients the burden of a potentially toxic treatment.
Shown below is an example of an assay measuring the concentration of a candidate stromal activation marker in the serum of patients treated in our hospital. A darker yellow indicates a high activation status, or amount, of stroma in a cancer patient. We are currently testing how well this assay predicts the response to novel stroma-targeting drugs in pancreatic and esophageal cancer.

RESISTANCE MECHANISMS
With the advent of targeted therapies, the expectation has been that for most malignancies a relatively non-toxic but effective regimen can be developed. Unfortunately, it is now clear that many cancers are not sensitive to such agents (pancreatic cancer) or quickly become resistant following exposure to such agents (esophageal cancer). We have optimized in vitro approximations of most clinically relevant treatment schedules, including chemoradiation. This allows us to identify the resistance mechanisms responsible and by querying next-generation sequencing databases, identify targetable molecules to thwart this resistance.
Hedgehog proteins AND cancer
Many events that shape the developing embryo are regulated by the so-called Hedgehog proteins. In the adult body, activity of these proteins is limited, but reactivation of the pathway is thought to contribute to the genesis and progression of several cancers, the most lethal of which is pancreatic cancer. Several lines of research in our lab deal with the production and transport, but also the reception and interpretation of this ligand. Most of our experiments on the interaction between tumor and stroma cells take place in a culture dish. The functional outcomes of culturing these cells in close proximity as they do in a real tumor can for instance be measured by using fluorescent (light-emitting) reporters.
For instance, in the microscope image below, cells representing the stroma light up green when they receive Hedgehog from the cancer cells that are labeled with a red color. What this enables us to do is quantitatively assess the spread of stroma-activating ligands from cancer cells and study the mechanisms responsible for this dissemination.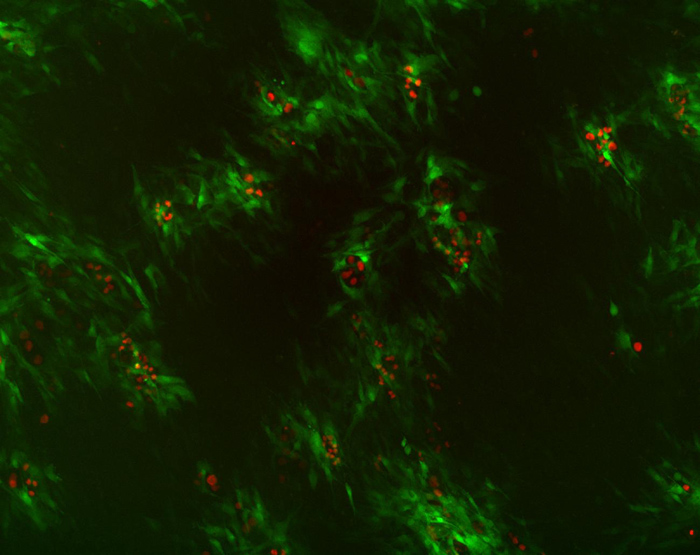
BioPAN and bi-oes
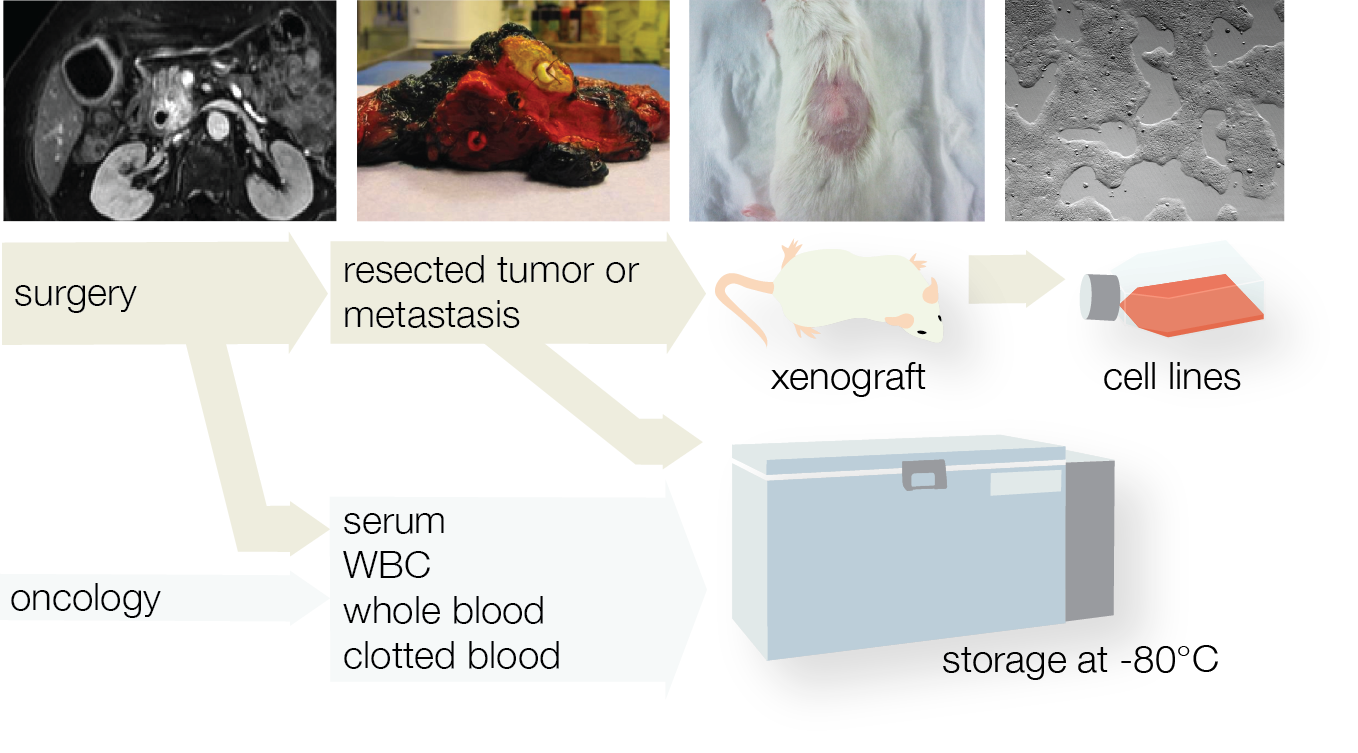
To construct the relevant experimental models needed to study pancreatic and esophageal cancer, and validate findings from experimental work, our lab has founded the AMC biobank pancreatic carcinoma – BioPAN and the biobank esophageal carcinoma – BiOES. For this biobank we accrue and store material like blood samples, tissue specimens, and clinical data. Analyses on these materials include expression profiling, measurements of serum markers (see above). BioPAN closely collaborates with the nation-wide PancreasParel biobank.

MOLECULAR SUBTYPES IN GI CANCERS
Our expression analyses of many hundreds of patients (PDAC, EAC, CRC) have enabled us to identify groups of patients, or rather their tumors, that differ in their growth, possibly drug response, and other important parameters in the biology underlying tumor development. In close collaboration with the Department of Pathology, we are also including multiple biopsies per tumor in these analyses to assess the heterogeneity that exists within tumors.
It is becoming increasingly clear that tumors from different organs share many similarities at the molecular level and that this offers an opportunity to consolidate and simplify disease classification. Work in the lab is now addressing these commonalities and exposing therapeutic vulnerabilities between cancers.
In addition, we attempt to graft tumor pieces from most patients in mice to create tumor models that faithfully mimic the patient tumor. All this research is done in strict accordance with European ethical legislation. Please also see our section on mouse work.
cancer stem cells and clonal dynamics
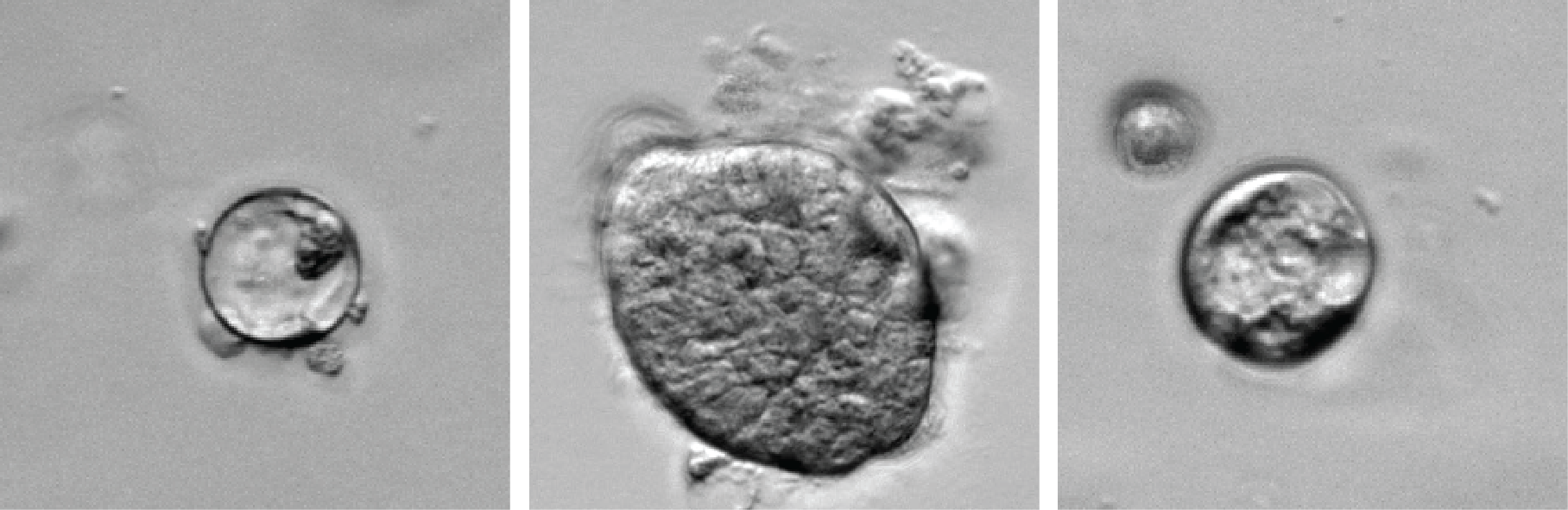
Many aspects that drive the lethality of upper GI tract cancer like its high resistance to treatment and aggressive growth pattern, rely on the presence of so called cancer stem cells. Because of their assumed importance for disease outcome, these cells have become the subject of an intense research effort. However, the tools to identify these cells -markers- have serious shortcomings. We have adapted a tracing methods that allow us to trace the offspring of a single cell in tumors grown from these cells. This then gives a handle, or tool, to study the population of cells that are thought to contribute to aggressive cancer growth. In addition, we have adapted cell culture methods that are suspected to maintain the activity of such cells:
Shown below are microscope images of so-called organoid cultures established from different patient tumors. Such cultures are thought to hold great promise as experimental and drug-testing platforms in addition to mouse work and traditional tissue culture.